
A team of researchers from China, Germany and the U.S. has developed a way to create a less fragile diamond. In their paper published in the journal Nature, the group describes their approach to creating a paracrystalline diamond and possible uses for it.
Prior research has shown that diamond is the hardest known material but it is also fragile—despite their hardness, diamonds can be easily cut or even smashed. This is because of their ordered atomic structure. Scientists have tried for years to synthesize diamonds that retain their hardness but are less fragile. The team has now come close to achieving that goal.
Currently, the way to create diamonds is to place a carbon-based material in a vice-like device where it is heated to very high temperatures while it is squeezed very hard. In this new effort, the researchers have used the same approach to create a less ordered type of diamond but have added a new twist—the carbon-based material was a batch of fullerenes, also known as buckyballs (carbon atoms arranged in a hollow spherical shape). They heated the material to between 900 and 1,300 °C at pressures of 27 to 30 gigapascals. Notably, the pressure exerted was much lower than is used to make commercial diamonds. During processing, the spheres were forced to collapse, and they formed into transparent paracrystalline diamonds which could be extracted at room temperature.
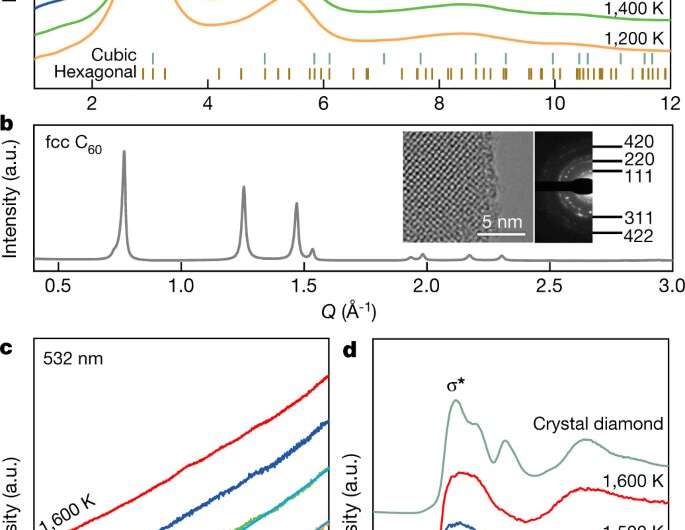
After making their less-ordered diamonds, the researchers looked at them under an electron microscope to learn more about their structure. They also subjected samples to X-ray diffraction and to atomist modeling. In so doing, they found their diamonds were made of disordered sp3-hybridized carbon, just as they expected. The goal of creating a less fragile diamond had been achieved. Unlike the results of another recent effort to synthesize a less fragile diamond, their resulting diamond is not completely amorphous (which would make it a type of glass), theirs is a type of amorphous diamond paracrystal. This means that it has a medium-range order—its atoms are ordered over short distances but not over long ones. Thus, no plane of atoms exist which means that the diamonds cannot be cut like natural diamonds.
© 2021 Science X Network
Citation: Creating a less fragile diamond using fullerenes (2021, November 28) retrieved 29 November 2021 from https://ift.tt/318ogtp
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
https://ift.tt/3FYZf2r
Science
No comments:
Post a Comment